CURCUMIN: IS IT ALL A LIE???
The actual TRUTH about Turmeric, Curcumin and other Curcuminoids.
If like me, you keep on top of current trends and research in the medical field and nutrition, then you probably know everything about turmeric (Curcuma longa), particularly curcumin.
You may even take curcumin on a daily basis, either because you believe it may offer some health benefit (as prevention) or some much-needed pain relief or to minimise the symptoms of some conditions (as a cure).
What I am about to tell you has been known for a long while, and yet the research has not been fully communicated across, probably because:
Manufacturers don't want to lose a market (maximise profit while the 'iron is hot'), where they are selling poorly-absorbable or just junk supplements (often laden with additives, fillers, and other nasties), using the cheapest raw ingredients. Some other manufacturers may use a formula made with raw ingredients that have been heavily processed in a way that the key constituents have been destroyed by heat, light, or various other processes and poor manufacturing methods. More importantly, compounds used in the formula may be extracted via the use of extremely toxic solvents. often derived from the petrochemical industry.
People may stop buying curcumin altogether, fearing that what they've purchased is virtually offering a placebo effect and nothing more.
It would propel ethical and customer-focussed companies ahead of the rest, providing health supplements that have been proven to be effective, and studied (the raw ingredients, the actual extracted compounds inside the capsule, bioavailability, and the actual activity inside the body/tissue/cell and efficacy. Is it really doing what the manufacturer claims the supplement does?) with properly-led peer-reviewed research.
The gold-standard (the usually 'accepted' type of study) is a randomised double-blind placebo clinical trial (a medical study, which involves human participants and neither side knows who's getting what treatment and placebo are given to a control group).[1]
Some companies do offer an indication of some studies being made by displaying the abstract from a particular study; however, this is only giving the title of the story. We all know that we should never judge a book by its cover. The same applies to research. We cannot judge a study by its abstract.
This is where it becomes complicated.
If you are not a researcher, a medical doctor or a trained practitioner, there is little you would understand from a study. Perhaps, the abstract will provide some kind of answers, but again, it would not be the full story.
The study may also be paid for or sponsored by a company for its own gain (e.g. nestle sponsoring a research paper to claim their chocolate is 'good' for health, when it is nothing but, and definitely not actual chocolate, but 'coloured' cocoa butter mass). Fair enough, this is just a non-factual example, but I hope it is helping you to better understand the difference between 'research' and 'research'...
So, is curcumin all that it is supposed to be?
It has been known for decades that the bioavailability of curcuminoids (the active constituents found in turmeric), including that of curcumin (only one type of active curcuminoid) is extremely low. By extracting curcumin from turmeric, the synergistic effects of all curcuminoids is also lost. To take an example, aspirin was originally extracted from willow bark (salicylic acid).[2] Today, aspirin is made in laboratories, and while it is an effective blood thinner and a potential painkiller, Aspirin is extremely destructive, particularly to the stomach lining. Long-term (or large) use of aspirin can cause bleeding in the stomach and the small intestine, but also in the brain and some other tissue, and lead to a wide variety of gastrointestinal symptoms.[3,4]
Extracting and using curcumin for therapeutic reasons must be proven by science for manufacturers to be able to make a claim. Indeed, there is large chunk of evidence demonstrating the anti-inflammatory and pain-killing properties of curcumin. However, there is even more research done to prove that the bioavailability of curcumin is extremely poor and that what is absorbed and used by the body is very low, even negligible ̶ and may provide very little therapeutic value, if none at all.
While some claims are legal (they must be approved by national/government agencies, and only made as directed), it may not apply to the product you are using. Yes, curcumin is anti-inflammatory and may be used for pain relief. And Yes, not all supplements are the same. And definitely yes, your supplement may be useless despite the claim on the label, because of all the reasons stated above (where was the original plant cultivated, the state of the soil? Was it grown organically and in a preserved area? Was it extracted and processed in a way that the active constituent remained intact? Is it encapsulated in a way that increases bioavailability? Has this particular product been shown to have any therapeutic effect on the body?).
Why this matters, would you ask?
Because research shows that curcumin is poorly absorbed by the body and the little that makes it through the cell membrane of the cells lining the gut wall, is glucuronidated almost immediately, which means that 'free' curcumin is attached to a glucose molecule and quickly excreted and little is made available to the cells of the body.
For example, research in the anticancer activity of curcumin has slow down dramatically because of the very poor bioavailability of curcumin (unless a megadose is administrated directly into the blood by a doctor). First because, the synergistic effect of all curcuminoids found in turmeric is lost, and so, reduces the bioavailability of curcumin. Second, because oral administration show negligible therapeutic effect.
“While some claims are legal, it may not apply to the product you are using.”
Some manufacturers claim that their supplemental form of curcumin is better absorbed and shows better result. Indeed, by adding piperine (a compound found in black pepper and ginger) extract to curcumin increases absorption (by about 200 times). This is amazing, right?
NO...!!!!
I repeat... NO!
Why?
Because the quicker curcumin is absorbed the quicker it is glucuronidated and the quicker it is excreted. NOTHING is nature works by cutting corners.
"There is no evidence that glucuronidated curcumin will cross the blood-brain barrier." explains Dr. Greg Cole, Professor of Medicine and Neurology as UCLA.
And so, it is back to square one.
To bypass this inconvenience, other manufacturers have created different formulae to increase the absorption and bioavailability of curcumin. And so, the market is now flooded with liposomal curcumin, BCM-95®CG curcumin, Longvida curcumin, and many more proprietary (patented) forms of curcumin supplementation.
“NOTHING in nature works by cutting corners.”
The studies.
Like I have said earlier in this article, the abstract given to substantiate the claims are available and given to resellers, and it is often a matter of 'copy-and-paste' the content from the abstract to the label. It is absolutely legal. After all, it is backed-up by research.
In my quest to understand curcumin extracts and the different forms of supplementation, and which is shown to have a greater therapeutic effect, the results have been extremely disappointing.
A meta-review of all curcumin extract manufactured/studied has been published just a few month ago (2020).(5)
Longvida® optimised curcumin.
The manufacturer claims that their patented curcumin extract is 65 times more available than standard curcumin and 285 times more bioavailable (how much is shown to circulate in the blood). Additionally, it says that its curcumin is able to circulate via the lymphatic system ('eradicating' the risk of glucuronidation altogether) and cross the blood-brain barrier.
The claim is based on the following study (clearly displayed on their scientific report):
Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. (2010). Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. Journal of Agriculture and Food Chemistry. 58(4), pp. 2095-2099. doi:10.1021/jf9024807. PMID: 20092313.
If you actually read the entire paper, you will be surprised to find out that the study was based on only 6 healthy male patients of Indian descent. Out of this minuscule group, 3 were made to
If you'd actually read the entire paper, you will be surprised to find out that the study was based on only 6 healthy male of Indian descent. This minuscule group was divided into two different groups, the control group (given a placebo) and the studied group (given 95% curcuminoids formula).
And so, the entire claim is made on 3 participants.
In the medical field, this type of study lacks credibility and would often be disregarded or used as a basis for further studies.
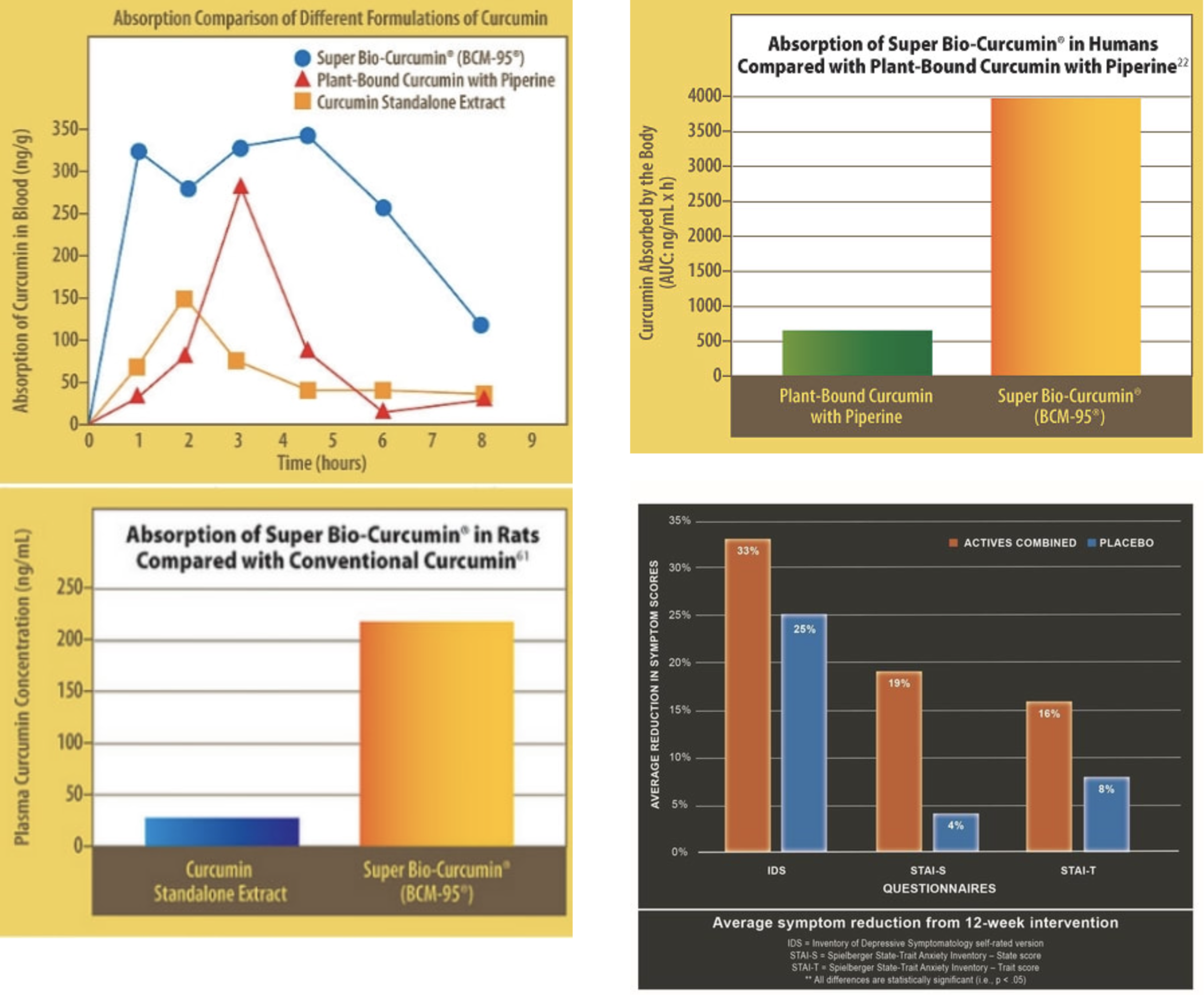
BCM-95®CG curcumin.
Results of a study indicated that "the relative bioavailability of BCM-95®CG (Biocurcumax™) was about 6.93-fold compared to normal curcumin and about 6.3-fold compared to curcumin-lecithin-piperine formula". Again 11 healthy subjects under the age group of 28–50 y were recruited for the study. They were divided in 3 different groups. 4 for Curcumin, 4 for control (placebo), and 3 (curcumin-lecithin-piperine formula).
And so, you might see on Bio-curcumin labels/bottles that it is "up to 7 times more bioavailable" than standard curcumin.
The study is. therefore, based on 4 volunteers.
The manufacturer claims that "BCM-95® (CURCUGREEN®) is the World’s most researched bioavailable formulated curcumin, manufactured under Arjuna’s international patents in world-class facilities by synergistic combination of curcumin, the active turmeric extract with Turmeric Essential oil specially enriched to 45% Ar-turmerone , another active turmeric extract . This makes for a powerful 95% bio activity enhanced curcuminoid complex which is 100% turmeric and 100% natural." Adding: "Safety of BCM-95® (CURCUGREEN®) is scientifically assured by an extensive study , the only one conducted so extensively for any bio-available curcumin which was done using in vivo and in-vitro models as per OECD guidelines."
BCM-95(R) Targets Anxiety and Depression (PRNewsFotoDolCas Biotech, LLC)
As per liposomal formulation, it appears that the complete array of curcuminoids offer the best approach to supplementation and therapeutic value.
So to make it easy, it is always best to look at a complete formulation and not only pure curcumin extract. It should be packaged in oil, preferably curcuminoid oil (or a mix of long-chain phospholipid (phospholipids are fat molecules used to make all cell membranes in the body, including brain cells. And so, might be readily-available and taken up by neurones), and long-chain lipid, to improve assimilation and potentially being carried via the lymphatic system and escape swift glucuronidation).
What's more?
Depending on your genetic make-up, you might absorb more or less than the average person, and so, to benefit from supplementation, you may have to take as much as your body requires.
Discuss this article with your doctor or practitioner, who may not be aware of the available research or what forms of curcumin is best for you and for what purpose.
If you feel nothing from the supplement you are taking, then perhaps this product is not for you.
References
Misra S. (2012). Randomized double blind placebo control studies, the "Gold Standard" in intervention based studies. Indian journal of sexually transmitted diseases and AIDS. (33(2), pp. 131–134. doi:10.4103/0253-7184.102130
Desborough, MJR. Keeling, DM. (2017). The aspirin story - from willow to wonder drug. British Journal of Haematology. 177, pp. 674-683. doi.10.1111/bjh.14520
Fanaroff, AC. Roe, MT. (2016). Contemporary reflections on the safety of long-term aspirin treatment for the secondary prevention of cardiovascular disease. Drug safety. (39(8), pp. 715–727. doi:10.1007/s40264-016-0421-1
Iwamoto, J. et al. (2013). Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy. World journal of gastroenterology. 19(11), pp. 1673–1682. doi.10.3748/wjg.v19.i11.1673
Stohs, SJ. et al. (2020). Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: A review. Molecules. 25, 1397. doi:10.3390/molecules25061397